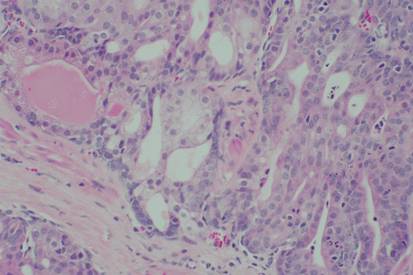
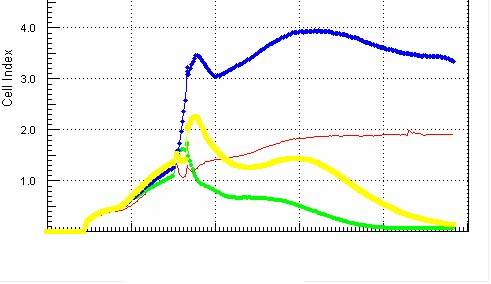
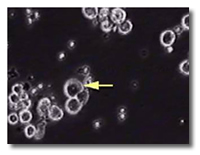
About G.I.F.T.
A UNIQUE APPROACH – GIFT and CKA
The GIFT treatment process, discovered by Professor Cui, MD/PhD and Professor Mark Willingham, MD, has revealed during initial and subsequent testing that;
• certain humans possess a genetically-defined and inheritable activity in their granulocytes to destroy a wide range of highly lethal cancers.
certain humans possess a genetically-defined and inheritable activity in their granulocytes to destroy a wide range of highly lethal cancers.
• this therapeutic activity can be transfused from cancer resistant donors to cancer-susceptible recipients for highly effective cancer treatment with minimal side effect.
this therapeutic activity can be transfused from cancer resistant donors to cancer-susceptible recipients for highly effective cancer treatment with minimal side effect.
Additionally, Munogenics also plans to commercialize a proprietary human test measuring CKA to determine an individual’s susceptibility, or resistance, to various types of cancers so that the risk for cancer can be managed or prevented. The CKA test will be used to identify potential donors for the GIFT cancer treatment process. Furthermore, the CKA test will measure granulocyte supplies in the following for humans:
• Selection for donors with high CKA (Cancer Therapy)
Selection for donors with high CKA (Cancer Therapy)
• Monitoring CKA in healthy population for cancer risk management (Prevention of Cancer)
Monitoring CKA in healthy population for cancer risk management (Prevention of Cancer)
• Monitoring CKA in cryo-stored cells (Aging Prevention)
Monitoring CKA in cryo-stored cells (Aging Prevention)
The CKA-based approach of detecting, and the GIFT therapeutic process for eliminating cancer relies on selecting specifically ready therapeutic reagents (human granulocytes) for direct use without any manipulation. The collection and transfusion process for human granulocytes has previously been approved by the FDA for human clinical use for other clinical indications, such as infections associated with neutropenia.
The CKA testing approach has been previously validated using a sampling of over hundreds of human subjects.
A volunteer population eligible for granulocyte donation for testing the GIFT treatment process has been identified.
GIFT consists of 4 major novel components which distinguish it from other cancer immunotherapies:
• Collects donor cells from correct cancer-resistant humans based on screening for high CKA, but not from random donors
Collects donor cells from correct cancer-resistant humans based on screening for high CKA, but not from random donors
• Collects and uses the specific types of donor cells which contain the highest CKA and minimizes potential side-effects
Collects and uses the specific types of donor cells which contain the highest CKA and minimizes potential side-effects
• Collects the donor cells at the precisely most effective time, since CKA is influenced profoundly by seasonality
Collects the donor cells at the precisely most effective time, since CKA is influenced profoundly by seasonality
• Collects sufficient number of granulocytes to achieve critical therapeutic doses
Collects sufficient number of granulocytes to achieve critical therapeutic doses
OVERSIGHT REQUIREMENTS AND APPROVAL STATUS
Munogenics and the Wake Forest team are prepared to immediately begin Phase 2
human clinical trials to discern which types of cancer can be cured by this
innovative, non-toxic means of treating various cancers.
The proposed clinical trials for GIFT require 2 parallel steps of regulatory approval:
• IRB (Institutional Review Board) approval
IRB (Institutional Review Board) approval
The GIFT IRB application was submitted on June 17th, 2007 at Wake Forest University. On July 27th, 2007 the IRB approved Wake Forest’s application for “White Cell Transfer as a Cancer Therapy”.
• FDA (Federal Drug Administration) approval for an investigative new drug (IND)
FDA (Federal Drug Administration) approval for an investigative new drug (IND)
After extensive communication with the FDA and the WFU research team, it is generally agreed that GIFT as an investigator-initiated clinical trial for an experimental therapy would NOT be categorized as an IND, as the process of granulocyte infusion has previously received approval. The FDA has approved an IND exception to WFU as the GIFT process does not manipulate donor cells in any way after collection at FDA-approved American Red Cross blood collection facilities, thus allowing for accelerated approvals.
PRESS COVERAGE AND ACCOLADES
Dr. Cui and the Wake Forest team of researchers have received considerable press validating their novel discoveries from detailed research. A sampling:
• Discover Magazine, “Are You Immune to Cancer?”
Discover Magazine, “Are You Immune to Cancer?”
• ScienCentral, “Cancer Curing Blood”
ScienCentral, “Cancer Curing Blood”
• Additionally:
Additionally:
o Forbes.com,
Forbes.com,
o LA Times,
LA Times,
o Scientific American,
Scientific American,
o BBC News,
BBC News,
o NY Times,
NY Times,
o Voice of America,
Voice of America,
o and others have provided significantly positive coverage.
and others have provided significantly positive coverage.